Cleanroom Classification for Aseptic Processing
Cleanroom Classification Recommendations for Aseptic Processing / Sterile Environments:
Critical Area – ISO 5 (Class 100) FDA Recommendations The critical area is where the sterilized drug product, as well as any containers and closures are exposed to environmental conditions that must be designed to maintain product sterility (§ 211.42(c)(10)). Activities conducted in such areas include manipulations (e.g., aseptic connections, sterile ingredient additions) of sterile materials prior to and during filling and closing operations. Source Sterile compounding guidelines vary depending on several factors. Configurations for sterile to sterile hazardous drug (HD) compounding will differ from those for non-sterile to sterile hazardous drug (HD) compounding. The following guidelines are taken from the proposed USP Chapter 800.
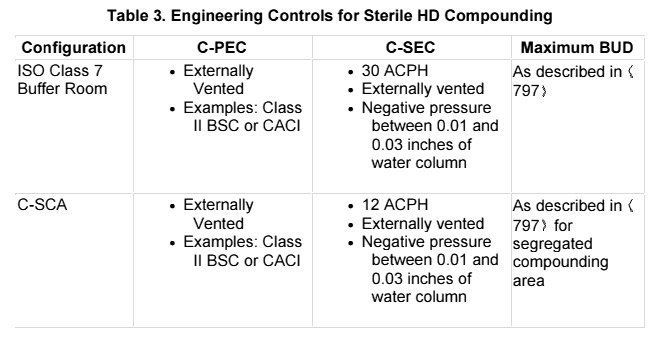
Buffer Area - ISO 7 (Class 10,000) from Sterile Compounding Guidelines for Proposed USP 800 The buffer area is a room that surrounds the space where sterile compounding is performed, providing an additional barrier of protection from the natural environment. It is recommended that a C-PEC is housed in an ISO Class 7 buffer room that has a negative pressure between 0.01 and 0.03 inches of water column and has a minimum of 30 ACPH of HEPA-filtered supply air. Because the room through which entry into the HD buffer room (e.g., ante-area or non-HD buffer room) plays an important role in terms of total contamination control, the following is required:
- Minimum of 30 ACPH of HEPA-filtered supply air
- Maintain a positive pressure of 0.02 inches of water column relative to all adjacent unclassified spaces
- Maintain an air quality of ISO Class 7 or better